Abstract
Background. Behçet's disease (BD) or syndrome is a rare chronic multisystem inflammatory condition with most manifestations attributed to vasculitis, including ulceration, skin lesions, with joint and other organ involvement. The poorest prognosis is reported in a small subgroup of BD patients with vascular, neurological, ocular, and gastrointestinal (GI) manifestations. The main aims of treatment are to suppress inflammatory exacerbations and recurrences, to prevent irreversible organ damage.1 There are few trial data supporting treatment options because of disease rarity and clinical heterogeneity. There are no previous or current clinical trials of hematopoietic stem cell transplantation (SCT) for BD, with only a few individual reported cases.
Objective. To assess the safety and efficacy of SCT with the research question: "what is the overall survival and progression free survival (PFS) of patients with BD post autologous SCT (autoSCT) and allogeneic SCT (alloSCT), including those with the primary indication of refractory disease".
Design. Systematic review of reported cases, with updated data for 4 patients treated at our centre in Liverpool, UK.
Data sources: PubMed, PubMed Central, Web of Science, OvidSP, Cochrane central register of controlled trials (CENTRAL), clinicaltrials.gov, eudract.ema.europa.eu, controlled-trials.com, free Google search, relevant conference proceedings, hand searching of reference lists, and contact with authors as necessary. Search terms were "behcet" and "transplant". The search was last updated on 31 July 2018.
Eligibility criteria. Cases of BD treated with autoSCT or alloSCT with available information on survival and PFS.
Data extraction. Two reviewers independently assessed articles for inclusion, extracted data to assess patients, interventions, comparisons, and outcomes.
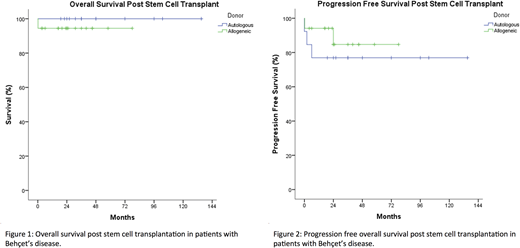
Primary outcome measures. PFS and overall survival.
Results. 32 patients with BD were reported in 25 identified articles, from Europe (47%), Asia (47%), and North America (6%). Median age was 36 years (range 6-60 years old), with 53% females, and 75% had had poor prognosis BD manifestations including vascular, neurological, ocular, and GI. Median time from BD diagnosis to SCT was 48 months (range 3-132), with a median of 3 (range 0-8) prior lines of BD-directed immune therapy.1 The primary indication for SCT was refractory BD in 41% of patients, and 59% of SCTs were primarily for other hematology indications. AlloSCT accounted for 59% of BD patients, the rest being autoSCT. Overall survival was 97% (Fig 1), after a median follow up of 31 months (range 0.2-135 months), with no difference between SCT types (p=0.36). The only death was from infection and sepsis prior to engraftment post alloSCT. ORR was 93%, and PFS was 112 months (95% CI 94-130) (Fig 2), with no difference by SCT donor types (p=0.46).
Refractory BD was the main indication for autoSCT in 85%, and myeloma accounting for the remainder. Reported autoSCT conditioning regimens included melphalan 140-200 mg/m2 (54%), carmustine/etoposide/cytarabine/melphalan (BEAM) (23%), and cyclophosphamide combinations (23%), with 46% incorporating additional anti-thymocyte globulin (ATG). OOR was 92%, with complete remission (CR) achieved in 62%, partial remission (PR) in 31%, and stable disease (SD) in 8%.
Refractory BD was the main indication for 11% of alloSCTs, and 5% had relapsed post autoSCT. Other hematology primary indications accounted for 89% of alloSCTs, with 69% for MDS, 11% AML, and 11% aplastic anemia. Trisomy 8 was recorded in 59%. Myeloablative conditioning was reported in 58%, with 26% using total body irradiation (TBI). OOR was 94%, with CR accounting of 84%. Acute or chronic graft versus host disease (GvHD) requiring therapy was poorly reported and lower than expected at 32%, with grade III-IV acute GvHD of 11%.
Conclusion. SCT in BD appears to be safe and effective, with no clear difference in long-term survival or PFS by SCT type. AutoSCT is a beneficial treatment option for refractory BD. AlloSCT is also advantageous in BD, with less data in the refractory BD or post autoSCT salvage settings. Given the lack of a clinical trial, we present the most comprehensive evidence available supporting the practice changing role of SCT as a treatment option in BD.
References. 1. Hatemi G, et al. 2018 update of the EULAR recommendations for the management of Behçet's syndrome. Annals of the Rheumatic Diseases 2018;77:808-18.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal